Publications
Highlighted Publications
Incident Major Depressive Disorder Predicted by Three Measures of Insulin Resistance: A Dutch Cohort Study
Watson KT, Simard JF, Henderson VW, Nutkiewicz L, Lamers F, Nasca C, Rasgon N, Penninx BWJH
Am J Psychiatry. 2021; Online ahead of print.
Major depressive disorder is the leading cause of disability worldwide. Yet, there remain significant challenges in predicting new cases of major depression and devising strategies to prevent the disorder. An important first step in this process is identifying risk factors for the incidence of major depression. There is accumulating biological evidence linking insulin resistance, another highly prevalent condition, and depressive disorders. The objectives of this study were to examine whether three surrogate measures of insulin resistance (high triglyceride-HDL [high-density lipoprotein] ratio; prediabetes, as indicated by fasting plasma glucose level; and high central adiposity, as measured by waist circumference) at the time of study enrollment were associated with an increased rate of incident major depressive disorder over a 9-year follow-up period and to assess whether the new onset of these surrogate measures during the first 2 years after study enrollment was predictive of incident major depressive disorder during the subsequent follow-up period.
Three surrogate measures of insulin resistance positively predicted incident major depressive disorder in a 9-year follow-up period among adults with no history of depression or anxiety disorder. In addition, the development of prediabetes between enrollment and the 2-year study visit was positively associated with incident major depressive disorder. These findings may have utility for evaluating the risk for the development of major depression among patients with insulin resistance or metabolic pathology.
Read the full article at The American Journal of Psychiatry »
Fibroblast growth factor 9 is a novel modulator of negative affect
Aurbach EL, Inui EG, Turner CA, Hagenauer MH, Prater KE, Li JZ, Absher DM, Shah N, Blandino P Jr, Bunney WE, Myers RM, Barchas JD, Schatzberg AF, Watson SJ Jr, Akil H
PNAS. Epub 2015 Sep 2.
Both gene expression profiling in postmortem human brain and studies using animal models have implicated the fibroblast growth factor (FGF) family in affect regulation and suggest a potential role in the pathophysiology of major depressive disorder (MDD). FGF2, the most widely characterized family member, is down-regulated in the depressed brain and plays a protective role in rodent models of affective disorders. By contrast, using three microarray analyses followed by quantitative RT-PCR confirmation, we show that FGF9 expression is up-regulated in the hippocampus of individuals with MDD, and that FGF9 expression is inversely related to the expression of FGF2. Because little is known about FGF9’s function in emotion regulation, we used animal models to shed light on its potential role in affective function. We found that chronic social defeat stress, an animal model recapitulating some aspects of MDD, leads to a significant increase in hippocampal FGF9 expression, paralleling the elevations seen in postmortem human brain tissue. Chronic intracerebroventricular administration of FGF9 increased both anxiety- and depression-like behaviors. In contrast, knocking down FGF9 expression in the dentate gyrus of the hippocampus using a lentiviral vector produced a decrease in FGF9 expression and ameliorated anxiety-like behavior. Collectively, these results suggest that high levels of hippocampal FGF9 play an important role in the development or expression of mood and anxiety disorders. We propose that the relative levels of FGF9 in relation to other members of the FGF family may prove key to understanding vulnerability or resilience in affective disorders.
Read the full article at PNAS »
Circadian patterns of gene expression in the human brain and disruption in major depressive disorder
Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ, Akil H, Bunney WE
PNAS. 2013 Jun 11;110(24):9950-5

A cardinal symptom of Major Depressive Disorder (MDD) is the disruption of circadian patterns. Yet, to date, there is no direct evidence of circadian clock dysregulation in the brains of MDD patients. Circadian rhythmicity of gene expression has been observed in animals and peripheral human tissues, but its presence and variability in the human brain was difficult to characterize. Here we applied time-of-death analysis to gene expression data from high-quality postmortem brains, examining 24-hour cyclic patterns in six cortical and limbic regions. Cyclic patterns were much weaker in MDD brains, due to shifted peak timing and potentially disrupted phase relationships between individual circadian genes. This is the first transcriptome-wide analysis of cyclic patterns in the human brain and demonstrates a rhythmic rise and fall of gene expression in regions outside of the suprachiasmatic nucleus in control subjects. The description of its breakdown in MDD suggest novel molecular targets for treatment of mood disorders.
Read the full article at PNAS »
Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals
Turner CA, Clinton SM, Thompson RC, Watson SJ Jr, Akil H
PNAS. 2011 May 10; 108(19):8021-5

The fibroblast growth factor system has been previously implicated by the Pritzker Consortium to be altered in the human post-mortem brain of individuals with Major Depressive Disorder (MDD). We have also shown that one of the members of this family, FGF2, has antidepressant and anxiolytic properties in rodents. These effects are thought to be due to an increase in the survival of cells in a region of the brain known to undergo new cell birth, the hippocampus. Recently, we decreased expression of FGF2 in this brain region, as illustrated at right. This manipulation resulted in increased anxiety in an animal model used to screen anti-anxiety agents. Taken together, these results suggest that levels of FGF2 in the brain not only modulate anxiety but can also produce anxiety.
Read the full article at PNAS »
Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior
Eren-Koçak E, Turner CA, Watson SJ, Akil H
Biol Psychiatry. 2011 Mar 15; 69(6):534-40
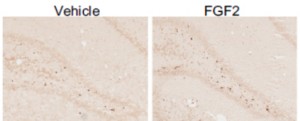
The fibroblast growth factor system has been implicated in the pathophysiology of mood disorders in humans and in affective behavior in animal models. However, the studies have been either correlative or involved exogenous administration of fibroblast growth factor 2 (FGF2). None of them have directly linked endogenous FGF2 to changes in emotional responses. Therefore, we began a series of studies to knockdown FGF2 by RNA interference to examine the role of brain FGF2 in emotional responsiveness.
The FGF2 knockdown in the hippocampus resulted in an anxiogenic effect. Together with our findings of an inverse correlation between anxiety and FGF2 expression levels, these results implicate FGF2 in the genesis and expression of anxiety disorders.